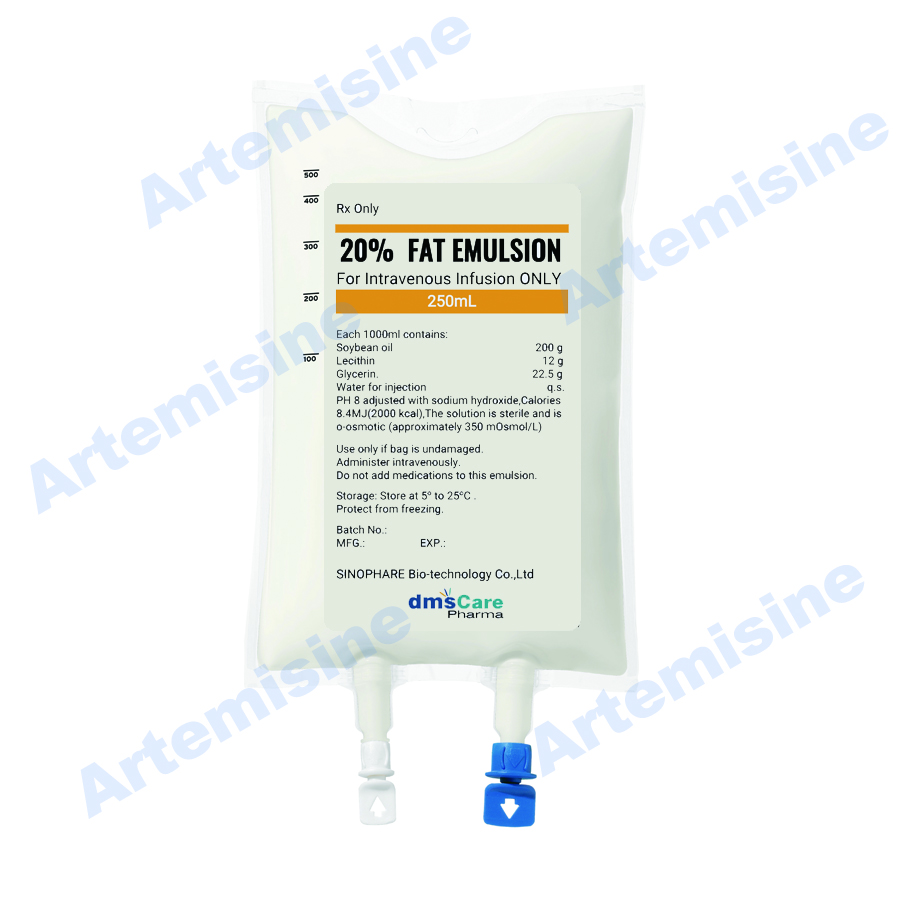
Product Description
Product Name:
20% Fat Emulsion Injection
Contains:
20% fat emulsion
Pakcage:
100 ml
Indications and Usage for Fat Emulsion
20% Fat Emulsion Injection is indicated as a source of calories and essential fatty acids for parenteral nutrition and as a source of essential fatty acids when a deficiency occurs when oral or enteral nutrition is not possible, insufficient, or contraindicated.
20% Fat Emulsion Dosage and Administration
20% Fat Emulsion Injection Pharmacy Bulk Package is not intended for direct intravenous administration.
For intravenous infusion through a peripheral or central line
Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and consideration of additional energy given to the patient.
For information on the age-appropriate infusion rate, see the full prescribing information.
|
Age |
Nutritional Requirements | |
| Initial Recommended Dosage | Maximum Dosage | |
| Preterm and term infants (<1 year) | 1 to 2 g/kg/day | 3 g/kg/day |
| Pediatric patients 1 to 10 years | ||
| Pediatric patients 11 to <17 years | 1 g/kg/day | 2.5 g/kg/day |
| Adults | 1 to 1.5 g/kg/day | 2.5 g/kg/day |
Dosage Forms and Strengths
Injectable emulsion:
- 20% (50 g/250 mL)(0.2 g/mL) of lipid in 250 mL single-dose flexible bag
- 20% (100 g/500 mL)(0.2 g/mL) of lipid in 500 mL single-dose flexible bag
- 20% (200 g/1,000 mL)(0.2 g/mL) of lipid in 1,000 mL Pharmacy Bulk Package
Contraindications
- Known hypersensitivity to egg, soybean, peanut, or any of the active or inactive ingredients.
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
Warnings and Precautions
- Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported.
- Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur, consider discontinuation or dosage reduction.
- Hypersensitivity reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
- Infections, Fat Overload Syndrome, and Refeeding Syndrome, Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters.
- Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Adverse Reactions/Side Effects
Adverse reactions have included hyperlipidemia, hypercoagulability, thrombophlebitis, thrombocytopenia .